Striving for improved genetic counselling & maternal supplementation
Characterising the Grainyhead-like 3 (GRHL3) Gene Regulatory Network in craniofacial development
La Trobe University
Assoc. Prof. Sebastian Dworkin

Slide title
Assoc. Prof. Seb Dworkin
Button
Slide title
La Trobe University
Button
Slide title
Dr Seb Dworkin and his team of researchers
Button
Craniofacial defects (CFD) are among the most common of all birth defects worldwide, affecting approximately 1-2% of all children to varying degrees. What makes these defects difficult to understand, categorise and treat, is that they are all so different to each other in terms of their severity, appearance and cause. These defects are typically only corrected with extensive, and often expensive, surgery once the child has been born. While this may improve the defect, it doesn’t treat the underlying cause – often genetic mutations in the parents – that increase the risk for future pregnancies (and therefore leads to affected siblings).
Funded by Craniofacial Australia, the primary goal of this research study is to better understand the nature, and function of genes that control formation of the head, face, brain, skull and jaws, and subsequently, how these may be overcome.
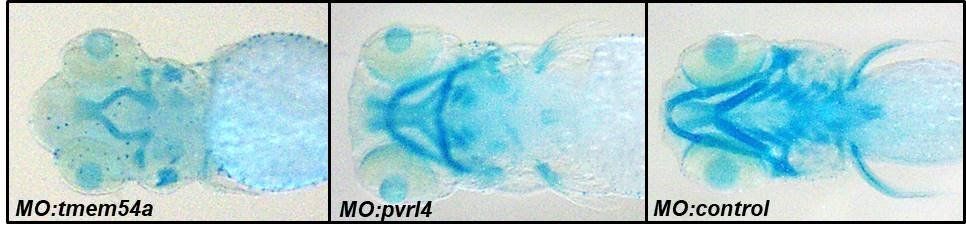
The team has identified that a single gene – termed Grainyhead-like 3 (GRHL3) GRHL3 is what’s known as a “master regulator” of craniofacial development; not just in humans, but in many different animal species as well (fruit fly, frog, fish and mouse). The intriguing thing about GRHL3 is that it’s only role appears to be to specifically control the function of other genes (known as target genes). To date, approximately 10 of these known target genes have already been implicated in CFD, indicating that characterising this critical “genetic network” will help us better understand genetic mutations that cause CFD before birth.
The team have identified a novel genetic pathway that leads to formation of the upper and lower jaws, and also the correct formation of the brain and spinal cord.
The team have begun to characterise the roles played by 7 potential GRHL3-target genes in the formation of the jaw. These genes have been implicated in multiple human craniofacial disorders, such as cleft lip and palate, Hartnup disorder, Seckel Syndrome, Jawad Syndrome and Pseudo-Torch Syndrome.
These studies are being further extended by using the cutting edge (literally!) technology of CRISPR, which “cuts” the DNA to introduce new genetic mutations at specific regions within a gene. The mutations are then passed down to offspring, allowing the team to identify the impact of these genetic mutations on future generations as well.
Lastly, testing is occuring on the effects of environment on embryos with a mutation in GRHL3.
The project will have two major impacts for future generations. Firstly, expanding the known genes that lead to craniofacial development will lead to significant improvements in genetic counselling. Genetic counsellors provide an essential, professional and empathetic service to prospective parents with either a family history of birth defects, or as a pre-emptive analysis of potential congenital anomalies in their newborns.
Secondly, to identify pharmacological compounds that may overcome these genetic mutations. We have a good understanding of harmful factors to avoid in pregnancy – alcohol, drugs, smoking, obesity, bacterial infections etc., but the spectrum of beneficial compounds is quite small. We know about the benefits of folate, magnesium sulphate and iodine supplementation, but beyond that, little is known. These factors could then be added to supplement the maternal diet of high-risk pregnancies, and reduce, or even remove, significant post-natal surgery.
204 Melbourne Street
North Adelaide SA 5006
Phone: (08) 8267 4128
Email: info@acmff.org.au
Sign up for our e-Newsletter
Welcome to the Craniofacial Australia family. We are thrilled to have you join this incredible craniofacial community of ours. At the centre are our families affected by a wide range craniofacial conditions, but an equally important part are our amazing donors, supporters, volunteers, researchers and medical professionals. Thank you for joining our community.
You will now receive our quarterly Newsletter – Changing Faces Changing Lives.
Don't forget to join our community on Facebook and Instagram, where you'll find regular updates on our craniofacial families and all the other happenings at the Foundation.
If you have any questions about our work or want to find out how you can get involved further, please do not hesitate to contact us direct.
If there’s scope to include a banner (such as the email footer banner below) which links to our donate page that would be great).
Many thanks,
Craniofacial Australia
P: (08) 8267 4128
Please try again later.
Registered Charity: CCP2573 | ABN: 29 008 155 780
All Rights Reserved | Craniofacial Australia
Web Design by Ignite Signs + Visual